This week McDonald’s announced it would cut its breakfast hours by an hour and a half to finish at 10.30 instead of 12.
The move has come due to an egg shortage caused by a bird flu outbreak. Southeast Australia has seen several strains of bird flu detected, with 11 poultry facilities affected in the past two months.
“Like many retailers, we are carefully managing supply of eggs due to the current industry challenges,” McDonald’s Australia said in a statement sent to the BBC.
“We’re continuing to work closely with our network of Aussie farmers, producers, and suppliers, as the industry comes together to manage this challenge.”
Authorities have assured that the situation is under control.
“Consumers can expect to see some empty shelves in the short term; however, supplies are being redirected to areas with shortages,” the Australian government stated.
“Consumers should refrain from purchasing more eggs than required.”
Bird flu has affected fewer than 10% of Australia’s egg-laying hens, but some businesses have imposed limits on egg purchases. The outbreaks have resulted in the culling of around 1.5 million chickens in Australia.
To date, none of the detected strains have been the H5N1 variant of bird flu. H5N1 has spread through bird and mammal populations globally, infecting billions of animals and a small number of humans.
Moderna to develop an mRNA vaccine
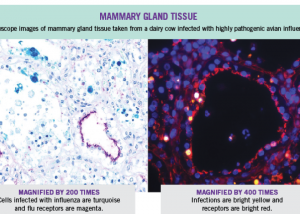
While the Australian government says it has the issue under control, the US government will pay Moderna Inc (NASDAQ:MRNA, ETR:0QF). $176 million to develop an mRNA vaccine against pandemic influenza, specifically targeting the highly pathogenic bird flu virus H5N1, which is currently spreading among US dairy cattle.
The funding is provided through the Biomedical Advanced Research and Development Authority (BARDA) as part of a new Rapid Response Partnership Vehicle (RRPV) Consortium. The initiative aims to establish partnerships with industry to better prepare for pandemic threats and develop medical countermeasures, according to a Department of Health and Human Services (DHHS) announcement on Tuesday.
Moderna stated that it began a Phase 1/2 trial of a pandemic influenza virus vaccine last year, targeting H5 and H7 bird flu viruses.
The company expects to release trial results this year, which will inform the design of a Phase 3 trial anticipated to begin in 2025. The funding will support late-stage development of a “prepandemic vaccine against H5 influenza virus,” and includes options for additional vaccine development if other public health threats arise.
“mRNA vaccine technology offers advantages in efficacy, speed of development, and production scalability and reliability in addressing infectious disease outbreaks, as demonstrated during the Covid-19 pandemic,” Moderna CEO Stéphane Bancel said.
“We are pleased to continue our collaboration with BARDA to expedite our development efforts for mRNA-based pandemic influenza vaccines and support the global public health community in preparedness against potential outbreaks.”
US health officials have previously indicated discussions with Moderna and Pfizer Inc (NYSE:PFE, ETR:PFE). about developing a pandemic bird flu vaccine. This future vaccine will complement standard protein-based bird flu vaccines.
Recently, the health department announced plans to manufacture 4.8 million vials of H5 influenza vaccine in response to the ongoing H5N1 dairy outbreak, which has exceeded initial containment expectations.
The US response to the outbreak has faced significant criticism from both domestic and international experts.
Genetic analyses suggest that the virus has been spreading among US dairy cattle since late last year. However, it wasn’t until March 25 that the US Department of Agriculture confirmed the first four infected herds in Texas and Kansas. Since then, the outbreak has spread to around 140 herds in 12 states.
Some farms are refusing to test, leading experts to believe there are many undocumented infections, especially given the detection of inactivated H5N1 in the commercial milk supply.
Among the 140 herds with documented infections, federal officials do not know how many are still actively infected. It remains unclear whether infected cows can be reinfected and, if so, how quickly.
While the general public is currently at low risk, farm workers face a higher risk of contracting the infection. To date, there have been three confirmed infections among dairy farm workers—one in Texas and two in Michigan, which has had a notably strong response to the outbreak.
Despite the risk to hundreds or thousands of farm workers, only 53 people in the country have been tested for H5 influenza.
You can now read the most important #news on #eDairyNews #Whatsapp channels!!!
🇺🇸 eDairy News INGLÊS: https://whatsapp.com/channel/0029VaKsjzGDTkJyIN6hcP1K