As bird flu spreads to cow herds in the US, the threat of similar outbreaks in Australia must be considered a high priority for governments.
The world is facing a new, concerning and fast evolving situation this year with the recent transmission of avian influenza A(H5N1) viruses (bird flu) to both dairy cows and humans in the USA. Prior to that, in just a few short years, massive numbers of poultry and mammals have become infected right around the world; the only unaffected continent so far is Australia.
This is not a great surprise, as influenza viruses constantly evolve and can spread easily in both wild birds and farmed poultry. Spillover to other animals (including humans) occurs when they have close contact with infected birds (dead or alive). Several avian influenza viruses are spreading globally and the most dangerous to humans at the moment are the H5N1 and H7N9 viruses.
Influenza viruses are classified into subtypes based on two groups of surface proteins: haemagglutinin (H), of which there are 18 (H1-H18), and neuraminidase (N) proteins, of which there are 11 (N1-N11). Influenza viruses are further classified by their disease severity as Low Pathogenic Avian Influenza (LPAI) or High (HPAI), based on their ability to cause disease in chickens.

USA on bird flu alert
So far in 2024, 191 dairy cow herds in the USA have been infected with H5N1 and since April 2024, at least 18 million birds, involving 35 commercial flocks and 20 backyard flocks, have tested positive for various influenza A(H5) viruses.
This year, there have been 14 human cases of avian influenza A(H5) infection in the USA; four associated with exposure to H5N1 infected cows, nine with exposure to infected poultry and one with no known occupational exposure to sick or infected animals, as reported by the US Centers for Disease Control and Prevention (CDC) in Atlanta. However, the CDC states that the present risk to the public from H5 bird flu remains low as currently there are no mutations allowing spread to humans.
Importantly, CDC is conducting a seroprevalence study in people exposed to the virus from cows and/or poultry; preliminary results show that none of the blood samples have evidence of infection (ie, avian H5N1 neutralising antibodies). This suggests that there is not considerable asymptomatic spread of the virus in humans, thus justifying CDC’s current risk assessment as low.
In addition, there have been no deaths, so far, among human (farm worker) H5N1 cases in the USA. Clearly, the bird/cow H5 flu viruses in the USA are not yet as virulent as has long been feared.
While influenza H5N1 virus does not transmit easily from birds/animals to humans or from person to person, it has previously caused severe disease with a high death rate. Since 2003, the H5N1 virus has caused 889 reported illnesses and reported 463 deaths in 23 countries, worldwide, with a case fatality rate of 52%.
Symptoms in humans include typical flu-like signs such as fever, cough, sore throat, vomiting, diarrhoea, and muscle aches; more severely, pneumonia, respiratory distress, and multi-organ failure. Currently, cases of bovine H5N1 virus in the USA are causing mild symptoms in humans including conjunctivitis — eye redness/irritation/discharge — and typical flu-like symptoms. There have also been many anecdotal (not laboratory confirmed) reports of conjunctivitis in workers on affected farms suggesting that there are many more human cases.
The origin and spread of influenza H5N1
Influenza H5N1 viruses first emerged in 1996, in Guangdong Geese, in China, and have continued to cause outbreaks in birds. However, since 2020 there has been an acceleration with an unprecedented number of deaths (millions) in wild birds and poultry across Africa, Asia and Europe. It caused a large outbreak in a mink farm in Spain with concern about mammalian spread.
In 2021–22, the virus spread to North America, killing hundreds of seals in New England and Quebec. The virus travelled further again in 2022, to Central then South America, even reaching the Antarctic over the past year.
H5N1 infections have now been detected in over 200 mammalian species: including domestic and wild animals such as bears (black, brown and polar), cats and dogs, foxes and minks, leopards and tigers; recently the virus has also been detected in alpacas, cows, elephants, elephant seals and sea-lions.
From birds to herds
The spread of the virus from birds into herds of dairy cattle in the USA has been concerning not only because of the immediate economic loss but also because it gives influenza viruses another avenue to mutate, for instance to recombine through co-infections with, and potentially better infect humans (and other animals); moreover, this evolution is likely to make it easier for the virus to transmit between humans. In addition, if bovine H5N1 flu becomes established (endemic) in cows, it would have long term consequences for the dairy and cattle industries and remain a threat to human health.
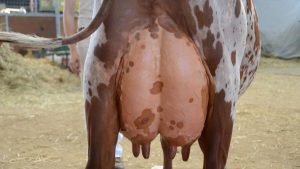
There is still some mystery around how this bovine H5N1 virus appeared and how it is spreading in dairy herds. It is known that the virus easily infects cow udders in dairy cattle and it is clear that milking machines have been spreading the virus from cow to cow, but some cows also have mild respiratory symptoms and nasal discharge, and the virus has been detected on muzzles and also in faeces, so it is likely that there are several routes of transmission from cow to cow with the potential to also infect humans.
The result is that milk production decreases markedly in cows and the milk is altered in colour and viscosity. Milk from infected animals contains a high level of virus but pasteurisation prevents viable virus from remaining in milk and stops transmission via milk to humans. There is a clear danger to those consuming unpasteurised milk. Effective pasteurisation and the destruction of milk from sick cows will ensure public safety.
How widespread is the virus in dairy herds in the USA? Surveys have shown that H5 fragments have been found in samples of commercially available milk in 38 states indicating that the infection is widespread, perhaps permanently circulating among cows and adapting efficiently to spread easily from cow to cow. It is unknown whether or not cows can be reinfected.
Stopping the spread
How can this bovine H5N1 be stopped? Large scale vaccination of cow herds might help to stop the spread. The use of mRNA vaccines is an option but yet to be tested and there are other vaccine types being developed, including recombinant protein vaccines and cell culture vaccines and microarray skin patches for delivery
In addition, there are some key measures that can be implemented including; strict biosecurity measures in poultry farms to prevent virus entry; maintaining good health practices in cattle herds, monitoring cattle for signs of illness and testing for viruses, educating farm workers and the public about hygiene practices and the risks associated with bird and animal contact, and, monitoring wild bird migration patterns as birds carry and spread the virus over long distances. Wastewater testing for bird and cow influenza viruses could also be implemented.
Can seasonal flu vaccines give protection against H5N1? No. But it can reduce the severity of seasonal flu. Importantly it could also reduce the risk of co-infection of a human influenza virus and an avian virus, which could lead to a new reassortant virus with pandemic potential. The public health concern about animal and bird influenza viruses lies in this possibility — that dual influenza virus infections could lead to a new influenza A virus, which may acquire the necessary receptor sites to allow efficient human-to-human spread.
Several countries have acted on H5N1 preparedness. The United Kingdom Health Security Agency recently raised its threat level and Europe has secured 40 million doses through the European Commission and is proactively vaccinating dairy and poultry workers against infection using vaccine from Australia’s CSL (AUDENZ™). The USA has a stockpile of H5N1 vaccines but is focusing on using seasonal flu vaccines for frontline workers as a way of preventing any reassortment of viruses and further mutation of H5N1.
What about Australia?
In Australia, during 2024, three different avian influenza strains (H7N3, H7N9, H7N8) are affecting poultry, which has led to the culling of hundreds of thousands of chickens. These new outbreaks of bird flu in Australia are in contrast with only 8 previous outbreaks in the previous 48 years. Human-to-human transmission has not yet been reported in Australia.
The CSIRO Australian Centre for Disease Preparedness notes that the H5N1 strain has been detected both to Australia’s north and south. Constant surveillance is important because as time goes by, the risk of H5N1 bird flu is increasing as it crosses into more wild animal species and livestock in other countries. Moreover, it is quite possible that birds from Asia will come into contact with infected migratory birds and bring the virus to Australia.
Given what is happening in the USA and the active preparedness plans for avian and bovine influenza in the UK, Europe and the USA, the threat to Australia must be considered a high priority for governments. It is clear that several factors make avian and bovine influenza viruses a potential candidate for the next pandemic, namely; the wide host range, the high mutation rate, the potential for genetic reassortment and global spread through migratory birds. Although many strains of avian flu are of low pathogenicity, these strains can evolve into highly pathogenic strains if they spillover from wild birds into poultry, as we’re seeing with the current outbreaks in poultry farms around the world, including Australia.
We need to be ready with vaccines, antiviral drugs, non-pharmaceutical and hygiene interventions right now and especially to avoid the complacency that happened with COVID-19 regarding poor vaccine (booster) uptake especially in the vulnerable, resulting in unnecessarily high death rates in aged care centres.
Gary Grohmann is a former director of immunobiology at the Therapeutic Goods Administration. He currently works as an independent consultant virologist. He is a board member and member of the scientific advisory committee of the Immunisation Coalition and an adjunct professor at the University of Sydney.
Robert Booy is an infectious diseases paediatrician. He is a Senior Professorial Fellow at the University of Sydney Children’s Hospital Westmead Clinical School. He is chair of the scientific advisory committee of the Immunisation Coalition.
You can now read the most important #news on #eDairyNews #Whatsapp channels!!!
🇺🇸 eDairy News INGLÊS: https://whatsapp.com/channel/0029VaKsjzGDTkJyIN6hcP1K